Wissenschaftliche Daten zu bariatrischer und metabolischer Chirurgie
-
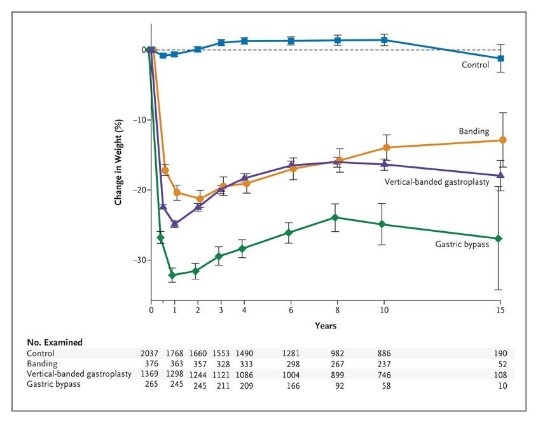
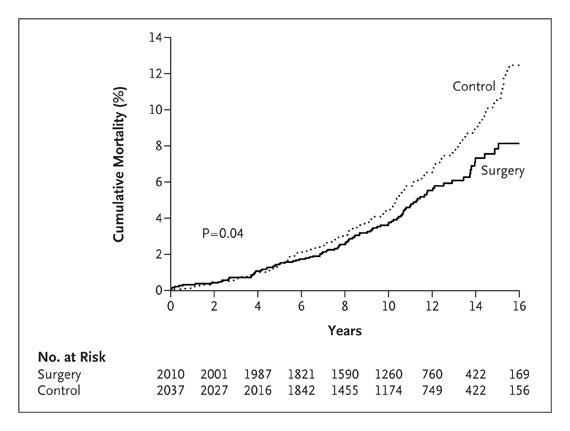
Die bariatrischen Operationen führen bei starker Adipositas zu langfristiger Gewichtsabnahme und Reduktion der Gesamtmortalität.
-
Figure 1. Mean Percent Weight Change during a 15-Year Period in the Control Group and the Surgery Group, According to the Method of Bariatric Surgery.
-
Figure 2. Unadjusted Cumulative Mortality. The hazard ratio for subjects who underwent bariatric surgery, as compared with control subjects, was 0.76 (95% confidence interval, 0.59 to 0.99; P=0.04), with 129 deaths in the control group and 101 in the surgery group.
-
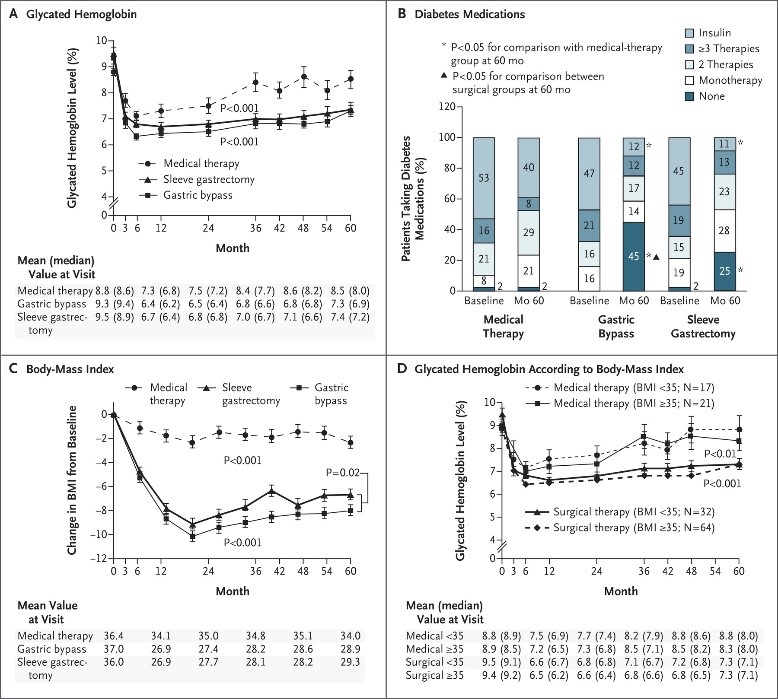
5-Jahres Ergebnisdaten zeigen bei Patienten mit BMI zwischen 27 und 43 dass bariatrische OP plus intensive medikamentöse Therapie effektiver ist in der Reduktion bzw. in einigen Fällen sogar Heilung von Hyperglykämie als intensive medikamentöse Therapie alleine.
-
Figure 1. Mean Changes in Measures of Diabetes Control from Baseline to 5 Years.
-
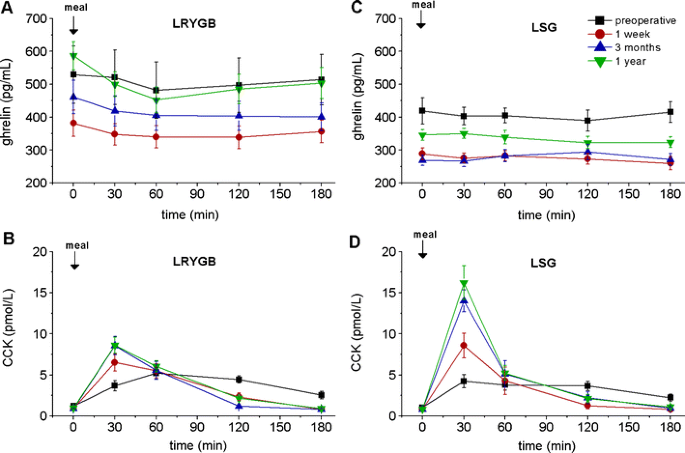
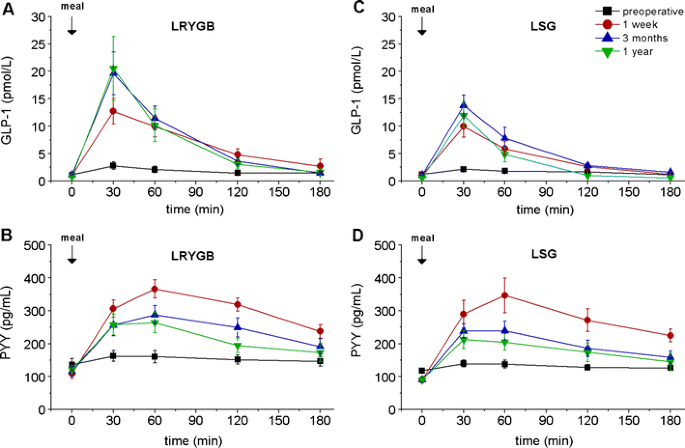
Bariatrische Operationen haben einen messbaren Effekt auf gastrointestinale Hormone, welcher auch einen weiteren Mechanismus für die Wirkung dieser Operationen darstellen könnte.
-
Fasting and meal-stimulated time courses of ghrelin and CCK in the two groups of patients (LRYGB and LSG) before, as well as 1 week and 3 and 12 months after the respective operation. a Ghrelin in the LRYGB group, b CCK in the LRYGB group, c ghrelin in the LSG group, d CCK in the LSG group
-
Fasting and meal-stimulated time courses of GLP-1 and PYY in the two groups of patients (LRYGB and LSG) before, as well as 1 week and 3 and 12 months after the respective operation. a GLP-1 in the LRYGB group, b PYY in the LRYGB group, c GLP-1 in the LSG group, d PYY in the LSG group.